The group’s research covers different theoretical aspects of so-called soft matter. According to Wikipedia, soft matter is “a subfield of condensed matter comprising a variety of physical systems that are deformed or structurally altered by thermal or mechanical stress of the magnitude of thermal fluctuations”. Being a truly interdisciplinary field in the crossroads between physics, chemistry and biology, soft matter science has impacts on such diverse fields as medicine, microbiology, food technology, and materials design. The set of methods employed by the group are mainly computational in nature, but much of the science is done in close collaboration with both experimental soft matter scientists and statistical physicists. Below are brief descriptions of the main current research interests of the group.
Research funding from the following organizations is kindly acknowledged

Collective behaviour of swimming microorganisms
Collaborators: Joost de Graaf (Edinburgh), Davide Marenduzzo (Edinburgh), Alexander Morozov (Edinburgh), Cesare Nardini (Cambridge), Rupert Nash (Edinburgh)
Many microorganisms, such as bacteria, algae and protozoa, live in aqueous environments, and thus have the need to actively self-propel (swim) through fluids. Depending on the details of their propulsion mechanism, each microorganism thus creates a fluid flow around it, which in turn affects both other swimmers as well as non-swimming (passive) particles in the solution. This “stirring” on the microscale has been shown to dramatically increase the mixing of passive particles, such as nutrients, compared to the predicted diffusivity stemming from pure thermal agitation. In addition, for rear-actuated microswimmers (so-called “pushers”) such as E. coli at high densities, a hydrodynamic instability occurs, leading to chaotic, collective motion over very large distances, so-called bacterial turbulence. In this project, we aim to understand these two collective phenomena through large-scale lattice Boltzmann simulations of microswimmers together with approaches from statistical physics.



Left: Schematic picture of a pusher, such as E. coli, and a puller, such as Chlamydomonas. The red arrows indicate the force coming from the self propulsion and the counterforce from fluid friction. Center-left: Movie of a simulated pusher suspension undergoing a transition to bacterial turbulence. Center-right: Fluid flow set up by a single puller swimmer in a spherical cavity. Right: Fluid velocity field in microswimmer suspensions below (left) and above (right) the transition to bacterial turbulence.
Physics of active Brownian particles
Collaborators: Mike Cates (Cambridge), Alexandre Solon (MIT), Julien Tailleur (Paris), Raphael Wittkowski (Münster)
In this project, we study a minimal model of so-called active matter, which denotes materials whose microscopic components are driven out of thermodynamic equilibrium. In particular, we consider materials composed of self-propelled particles, which include both synthetic (“catalytic microswimmers”) and biological (swimming bacteria and algae) examples. As a minimal model for active matter, we study so-called active Brownian particles (ABPs), which are self-propelled spherical particles that interact through a short-range excluded-volume interaction. In spite of being a very simplified model of active matter, ABPs have been shown to exhibit many intriguing features, including the ability to phase-separate into dense and dilute phases even in the absence of any attractive interparticle interactions, so-called motility-induced phase separation (MIPS). Using large-scale Brownian dynamics simulations together with accurate theoretical approaches from statistical physics, we aim to build a quantitative understanding of the non-equilibrium “thermodynamics” governing MIPS. In addition to MIPS, we study how active forces (self-propulsion) can be used to build new strategies for self-assembly of colloidal particles. These strategies using active particles for the well-controlled self-assembly of passive (non-swimming) colloids, and the use of light to spatially control the swim-speed of active particles in order to achieve the desired structures.

Left: A self-assembled “active ratchet” built from light-controlled active particles [Link]. Center-left and center-right: Active Brownian particles undergoing motility-induced phase separation into dense (yellow/red) and dilute (purple/blue) phases in 2 and 3 dimensions, respectively [Link]. Right: A “shock-wave” created by a collection of active particles surrounding a circular arrangement of non-swimming (passive) particles [Link].
Fluctuations and deformation of lipid vesicles
Collaborators: Håkan Wennerström, Emma Sparr
Every cell of all living organism is surrounded by membranes composed of lipid molecules that spontaneously self-assemble due to their amphiphilic character. Since the energy difference between various mesoscopic structures are small, meaning on the order of the thermal energy kBT, minute changes in conditions, such as temperature, solute concentrations, or lipid composition, can lead to drastic changes in membrane conformation with resulting changes in transport properties or membrane stability. In this project, we study models of spherical lipid vesicles and their response to changes in external solution conditions. In particular, when exposed to osmotic imbalances between the interior and exterior, spherical vesicles composed of a symmetric lipid bilayer are known to deform abruptly into a prolate shape for sufficiently high solute concentrations. In a recent paper, we however show that the measured deformation pressure differs by approximately 7 orders of magnitude from the theoretically predicted one from theories based on continuum elasticity. In a follow-up paper, we show that the inclusion of thermal fluctuations change the collapse transition from discontinuous to gradual, however without changing the predicted collapse pressure. Explaining this discrepancy between theory and experiments is therefore an ongoing research effort in the group.
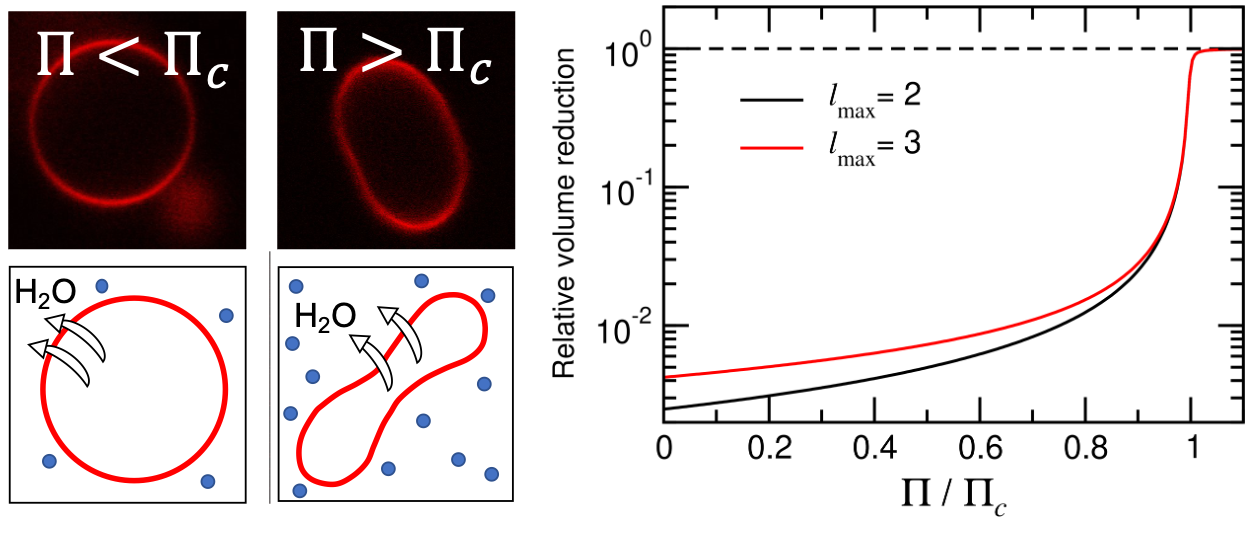 Left: When exposed to sufficiently large osmotic gradients, spherical vesicles deform into a prolate shape. [Link] Right: Relative volume reduction of a spherical vesicle as a function of the reduced osmotic gradient, showing that the inclusion of thermal fluctuations lead to a gradual collapse transition instead of the discontinuous one predicted in the athermal case. [Link].
Left: When exposed to sufficiently large osmotic gradients, spherical vesicles deform into a prolate shape. [Link] Right: Relative volume reduction of a spherical vesicle as a function of the reduced osmotic gradient, showing that the inclusion of thermal fluctuations lead to a gradual collapse transition instead of the discontinuous one predicted in the athermal case. [Link].